Shopping Cart [more]
Index of Products
Information
- 900 Series Chemical Process Control Case Studies
- Automatic Rinse Tank Controls
- Boilers and Cooling Towers
- Circuit Board Cleanliness Testing
- Deionized Water
- Environmental Applications
- Fountain Solutions
- Frequently Asked Questions
- Glossary of Terms
- Hemodialysis
- Horticulture
- Hydroponics
- Material Safety Data Sheets
- Operation Manuals / Instructions
- Oxidation Reduction Potential/Redox
- Pool and Spa
- Product Data Sheets
- Reverse Osmosis
- Textiles
Hemodialysis
Controlling dialysate quality is critically important to hemodialysis patient health. Complications as minor as nausea and fatigue or as severe as metabolic acidosis and sepsis can result if dialysate composition is incorrect. All the factors that ultimately affect dialysate composition must therefore be carefully monitored and controlled: proper proportioning and mixing of concentrates with water; the quality of water mixed with concentrates to form dialysate; and the quality of water used in the reprocessing of hemodialyzers, system maintenance and disinfection.
DIALYSATE QUALITY CONTROL
Dialysate preparation is specific to the dialysate type and formulation as indicated by the dialysate manufacturer and prescribed by the attending physician. Two types of dialysate are in current use: acetate and bicarbonate. Acetate is less popular today than bicarbonate and requires only dilution with water for final use. Bicarbonate is used most extensively and is made by mixing an acid concentrate (typically a liquid) with a bicarbonate concentrate (typically a powder) then diluting with water to form the final dialysate. Acid concentrates come in ionic concentrations specific to the type of bicarbonate concentrate they are mixed with. Bicarbonate concentrates can be comprised of sodium bicarbonate only or sodium bicarbonate mixed with sodium chloride to increase the ionic concentration.
Dialysate preparation is either done by the dialysate vendor or at the clinic. Solution temperature is a major impacting factor in preparation and must be maintained within the range prescribed by the dialysate manufacturer to achieve the proper mixture. Monitoring and controlling temperature is therefore necessary during solution preparation to ensure quality. The physical mixing of the dialysate solution also impacts final dialysate quality. Over mixing bicarbonate, particularly, will decrease carbon dioxide and increase pH. Solution conductivity is measured per manufacturer's specifications to ensure proper solution concentration, and measurement of solution pH and conductivity are both required as a final quality check.* A handheld instrument is ideal in this application where these parameters must be checked on the spot. The FDA-accepted D-6 and D-4† Digital Dialysate Meters™ were specifically designed to test dialysate quality. The D-6 measures Conductivity, pH and Temperature among other parameters, and the D-4 measures Conductivity and Temperature.
Checks of proper concentrate, conductivity, and pH should be included in the pretreatment check of all components and alarm systems of the dialysis machine. This is because problems in dialysate dispensing can and do occur due to human error. Clinics use varying types of dialysate, and different types of dialysis machines require different proportioning ratios. Lines can be crossed or an incorrect solution type selected, making it possible to administer a wrong dialysate. If the conductivity is off, the effectiveness of the treatment is reduced and patient electrolyte balance can be altered. It is also possible for concentrate dispensed by the dialysis system to be proportioned to the correct conductivity and yet have the incorrect pH, which can result in acidosis or alkalosis and ultimately death to the patient.
* Neither Conductivity nor pH alone is an infallible indicator of dialysate suitability. Both measurements are required for determining the proper mixture of dialysate. ANSI/AAMI RD52:2004 recommends that both conductivity and pH be checked prior to each treatment.
† The Myron L® Company Digital Dialysate Meter Models D-6 and D-4 have received FDA 510(k) acceptance for use as a medical device for testing dialysate. FDA Device Manufacturing License #s: 65387 (CA), 2020976 (Federal)
All dialysis systems currently on the market include an electrical conductivity sensor to monitor the mixture and to initiate action (e.g., alarms, flow bypass.) However, some dialysis systems do not include a pH monitor. An independent pH measurement is required to verify dialysate pH in those instances. Dialysate with a pH below 6.5 or above 7.5* is unsafe. Use the D-6 Dialysate Meter to check the pH and conductivity of the dialysate, as well as the conductivity, pH, and temperature alarm systems, before each dialysis treatment.† Myron L® D-4 and handheld analog D-1 and D-2 Dialysate Meters can also be used exclusively for checking dialysate conductivity. The D-4 measures temperature, as well.
* Standards for water quality and dialysate preparation in the U.S. were developed by the Association for the Advancement of Medical Instrumentation and approved by the American National Standards Institute, Inc. Water used to prepare concentrate must meet water quality requirements of ANSI/AAMI RD62:2006. Concentrate preparation and dialysate proportioning must meet the requirements of ANSI/AAMI RD52:2004. Examples given here are not a substitute for adherence to these standards or any other standards that apply to dialysis water quality, dialysate preparation, water treatment or dialysis system maintenance, and any other compliance requirement.
† Measurements of pH and Conductivity by an independent device do not supersede hemodialysis system readings. If there is a disparity in measurements between the independent device and the hemodialysis system, stop treatment and investigate the source of the disparity immediately.
WATER TREATMENT FOR HEMODIALYSIS
The quality of the water used in the mixing of dialysate and the maintenance of dialysis systems is also critically important. Chemical constituents of the water can change the ionic composition of the dialysate thus altering the concentration gradient in the dialyzer; react with constituents of dialysate or blood changing the chemical composition of the dialysate prescription or generating unwanted precipitates; or pass through the dialyzer membrane at levels toxic to the patient. During one week of treatment, a hemodialysis patient is exposed to 25 times the quantity of water (and thus 25 times the amount of contaminants) than an average person consumes in drinking water during the same period. Those contaminants are not filtered or excreted by the body as they are in normal consumption. In the dialyzer, solutes and water pass directly through a size-selective, rather than contaminant-specific, membrane into the bloodstream. Water from municipal facilities or other sources used for these purposes must, therefore, be carefully monitored and controlled to protect patient health. Accordingly, action levels and constituent maximums for water treated for hemodialysis and dialysate are now the same.
Configuration of a water treatment system is entirely up to the dialysis clinic, but should be done in consultation with the water supplier to ensure clinic treatment equipment will be able to handle worst-case contaminant levels determined by historical fluctuations in water quality. Standard treatment processes are dependent on the types of constituents that must be removed and the overall water quality coming from the supplier. For example, if feed water is high in TDS, DI systems alone will not be an economical solution for contaminant removal.
Care must be taken to order treatment processes such that a subsequent process does not undermine the treatment process(es) that preceded it. Each process must also be carefully monitored and controlled to ensure final product water quality and to maintain equipment function.
Carbon adsorption is typically used for the removal of chlorine disinfectant present in the feed water. Both free chlorine and chloramine levels must be controlled. The presence of these contaminants in hemodialysis solution could lead to hemolysis, resulting in death to patients. Removal of chlorine and chlorine compounds also prevents degradation of reverse osmosis (RO) filters used downstream in the water treatment system. In hemodialysis clinics, two carbon tanks are required. Carbon adsorption must be checked at the beginning of each treatment day, before each patient shift or every 4 hours, with samples being drawn after at least 15 minutes of operation. Samples are taken from testing ports following each tank, with action required if the second testing port is positive. The accidental use of expired reagents or test strips or ones not sensitive enough to detect unsafe chlorine levels can create errors in detection. The D-6 Dialysate Meter ORP function does not require the use of reagents or test strips and generates extremely accurate objective digital results.
pH, temperature, and the presence of other naturally occurring and synthetic organic compounds competing for active sites on the carbon profoundly affect the filter's efficiency. A high pH, the presence of N-chloramines and the use of orthophosphate or polyphosphate for corrosion control in distribution systems decrease the effectiveness of carbon in the removal of chloramines by adsorption. Feed water should be monitored for these conditions and adjusted accordingly by pretreatment.
In systems with automatic controllers, checks by independent devices, such as the D-6, are required to ensure proper system functioning. Use the D-6 or ULTRAPEN PT2 to monitor pH and determine the need for and effectiveness of acid injection or other pH adjustments. The D-6 and PT2 also measure and report temperature with every reading. A Myron L® 900 Series Multi-Parameter Monitor/Controller can be used to automatically adjust pH based on established setpoints.
A filter may follow carbon adsorption to trap carbon particles and then a softener downstream the filter to remove calcium from water to be processed by reverse osmosis. Though the D-6 and ULTRAPEN PT1 do not test for the presence of specific ions, testing for TDS using an NaCl salt solution characteristic is a good indicator of the quality of the water before it enters the RO system.
Water processed by reverse osmosis must be monitored by a continuous reading monitor for conductivity or Total Dissolved Solids (TDS) with a reliable alarm that can be heard in the treatment area. A divert to drain mechanism is also required. Use a 900 Series Multi-Parameter Monitor/Controller with an optional Remote Alarm to alert staff and divert water in the case that product water is outside of quality specifications.
Reverse Osmosis is usually followed by a deionization (DI) system as a backup or polisher. Carbon adsorption must precede DI with ultrafiltration (UF) afterward. If deionization is used for final removal of ions, water quality must be monitored continuously to produce water of 1 megohm/cm or greater resistivity at 25o C. Resistivity monitors are placed downstream of each bed. The system must also be monitored continuously for temperature by temperature-compensated resistivity monitors with an alarm audible in the patient care area. A divert to drain mechanism is required as a back-up for this system. The 750II Monitor/controller with optional audible alarm can be used in this application. Low pH may result when a deionizer is exhausted; pH should be monitored by an independent instrument and readings recorded twice each day. The D-6 is ideal for spot checks against automatic systems of control and can be used to measure resistivity and pH with the same degree of accuracy as many laboratory instruments measuring the same parameters.
If a DI system is used, it must be followed by UF to reduce bacterial/endotoxin contamination. UV irradiators and ozone generators can also be used for bacterial control. Ozone levels must be monitored in water with each use. The D-6 ORP mV function is designed to measure the total killing power of all oxidizing and reducing agents in solution and will precisely indicate the oxidizing power and hence the effectiveness of ozone treatment. A 720II Monitor/ controller can be used to continuously monitor the ORP of ozone-treated water. Use the D-6 Dialysate Meter ORP mV function to check against the functioning of automatic ORP Monitor/controllers or to manually monitor system functioning.
SYSTEM MAINTENANCE
Water quality should also be monitored during system maintenance. During routine low-level disinfection of the plumbing (to prevent build-up of biofilm) a check should be done to make sure no residual disinfectant remains in the system after disinfection. Testing for disinfectant in the rinse water should be done at the end of the disinfection loop. Dialysate mixing and storing systems must also be cleaned and maintained to prevent unwanted chemical or biological contaminants. Bicarbonate tanks, in particular, are susceptible to contamination and must be disinfected frequently. Use the D-6 to test for residual oxidizing germicides following disinfection of dialysis holding tanks, distribution systems, and machines using the ORP mV function. When verifying the complete removal of residual oxidizing germicides, such as chlorine, ORP mV reading should be as low as possible. Generally, an ORP reading of < 275 mV is a good indicator that there is very little residual oxidizer remaining. However, it is recommended that a target reading matching or close to the ORP value measured at the output of your RO system be sought.
Test concentrations of bleach solutions used for jug disinfection, as well, using the D-6 ORP free chlorine function.
In hot water disinfection systems the exposure time and temperature must be monitored at the point furthest from the water heater to ensure the system is exposed to water hot enough for a long enough period of time to control bacteria. Both the D-6 and D-4 precisely measure temperature via thermistor in the conductivity cell with every reading taken.
EQUIPMENT VERIFICATION
D-6 and D-4 Digital Dialysate Meters™ can also be used to verify hemodialysis equipment. Depending on applicable U.S. or European standards, the test accuracy ratio (TAR) required for calibration instrumentation can range anywhere from 3:1 to 4:1. Consult equipment manufacturer's accuracy specifications and ANSI/NCSL Z540-1-1994 Part I and ANSI/ISO/IEC 17025:2000 for requirements of equipment used for calibration, generally.
WASTEWATER MANAGEMENT
The D-6, D-4, PT1, PT2, and PT3 as well as 900 Series Multi-Parameter Monitor/Controller are also ideal tools for monitoring effluent (wastewater) quality in compliance with state, local and federal regulations.
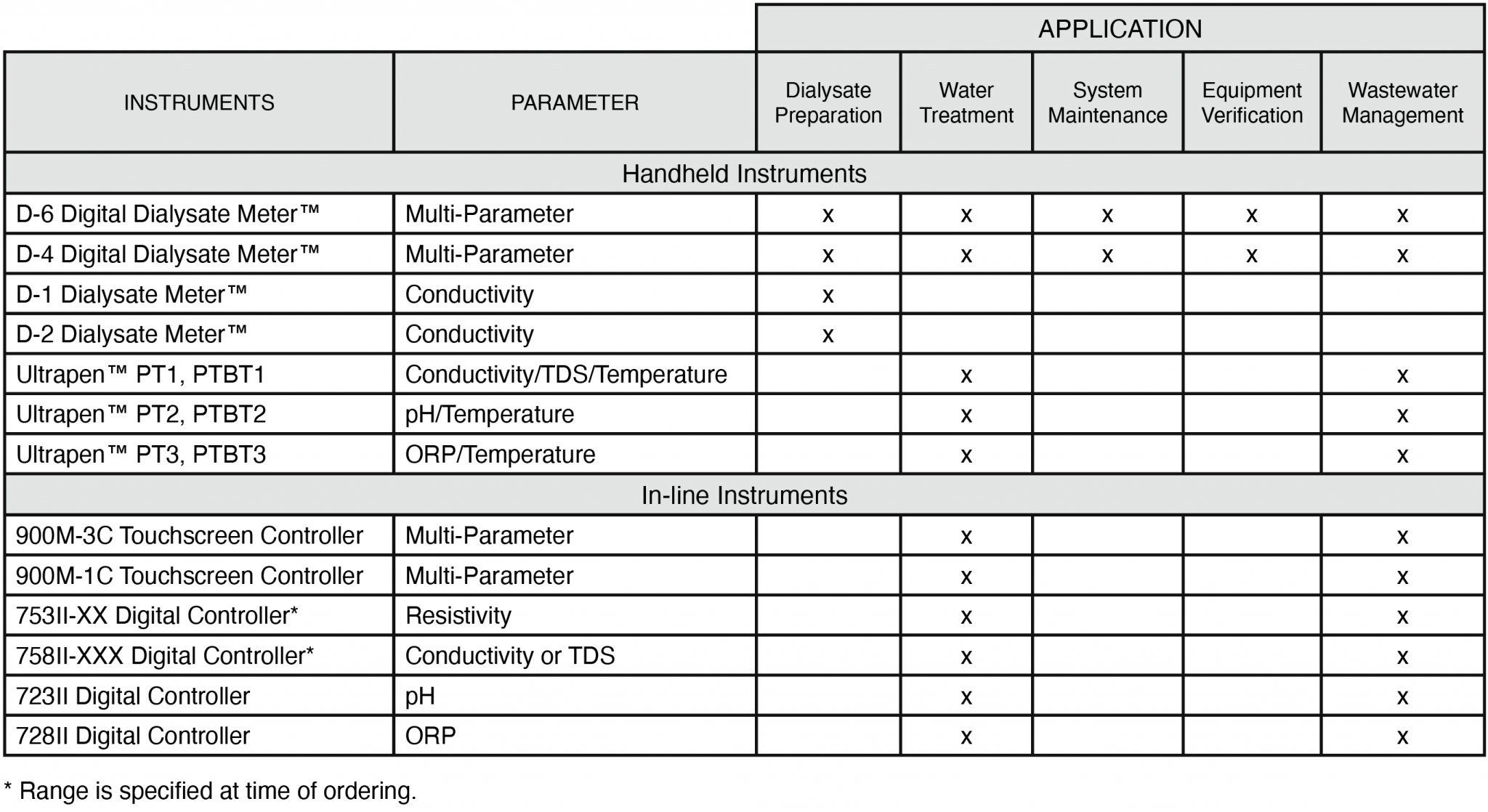
The following table briefly covers some of the Myron L® Company instruments recommended for common hemodialysis applications.
Other products and companies referred to herein are trademarks or registered trademarks of their respective companies.
**Prices, images and specifications subject to change without notice.**


For your convenience, we accept these credit cards: